Kansarka sambabada unugyada aan yarayn (NSCLC) ayaa qiyaastii ah 80%-85% wadarta tirada guud ee kansarka sanbabada, qalliinka dib-u-soo-celinta ayaa ah habka ugu waxtarka badan ee daaweynta xagjirka ah ee NSCLC hore. Si kastaba ha ahaatee, iyada oo kaliya 15% hoos u dhac ku yimid soo noqoshada iyo 5% horumarinta 5-sano ee badbaadada ka dib daaweynta kemotherabi qalliinka, waxaa jirta baahi caafimaad oo weyn oo aan la daboolin.
Immunotherapy Perioperative ee NSCLC waa goob cilmi baaris oo cusub sanadihii ugu dambeeyay, iyo natiijooyinka tiro ka mid ah wajiga 3 tijaabooyin la xakameynayo oo la kala soocay ayaa aasaasay booska muhiimka ah ee immunotherapy perioperative.
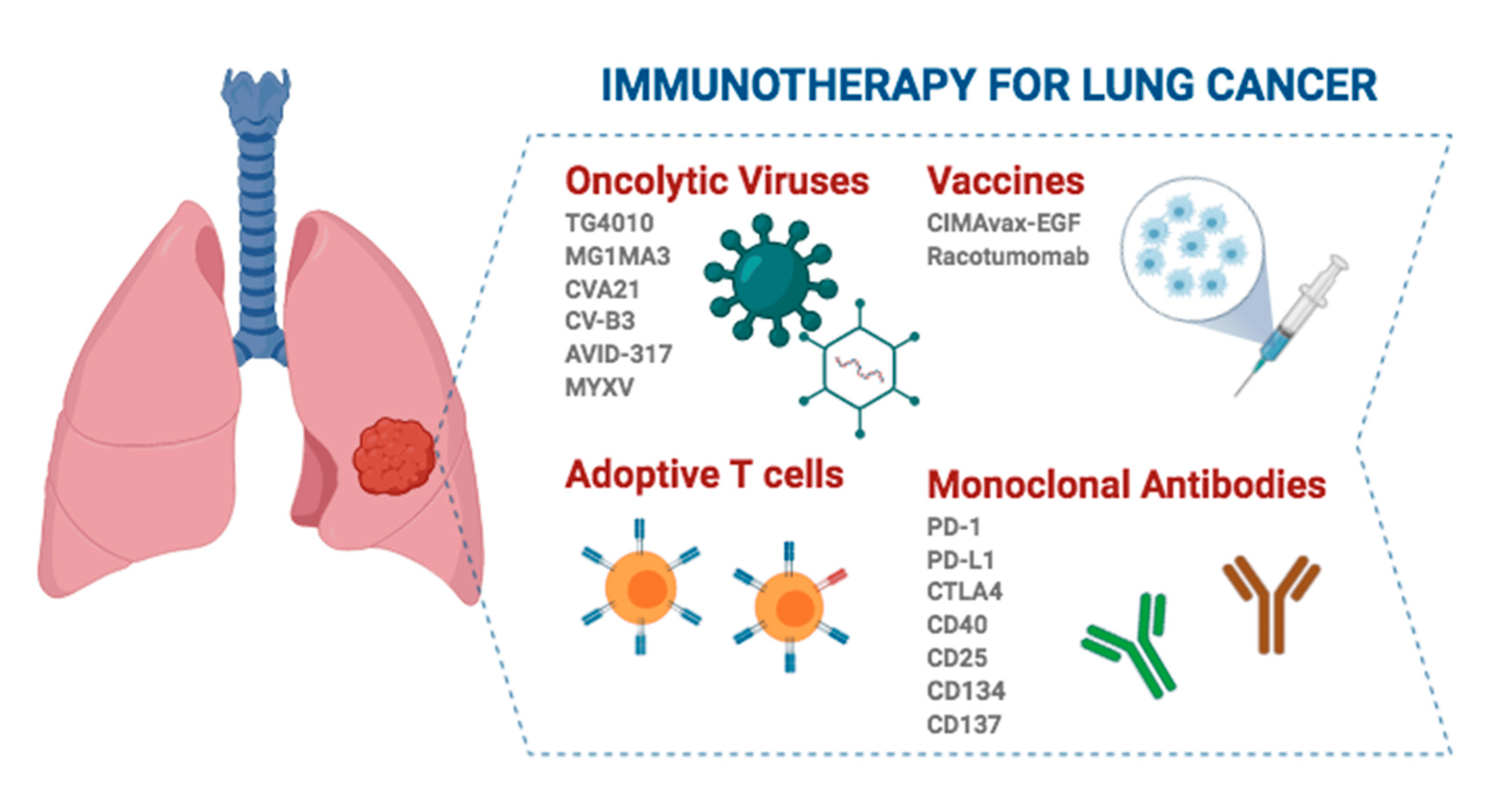
Immunotherapy ee bukaanada qaba kansarka sambabada unugyada yaryar ee aan yareyn (NSCLC) ayaa sameeyay horumar la taaban karo sanadihii la soo dhaafay, iyo istaraatiijiyadani ma aha oo kaliya inay kordhiso badbaadada bukaanka, laakiin sidoo kale waxay wanaajisaa tayada nolosha, iyada oo siinaya kabitaan wax ku ool ah qalliin dhaqameed.
Iyadoo ku xiran marka tallaalka difaaca jirka la bixiyo, waxaa jira saddex nooc oo tallaalka difaaca jirka ah ee daaweynta NSCLC ee marxaladda hore:
1. Neoadjuvant immunotherapy kali: Immunotherapy ayaa la sameeyaa ka hor qaliinka si loo yareeyo xajmiga burada loona yareeyo khatarta soo noqoshada. Daraasadda CheckMate 816 [1] waxay muujisay in tallaalka difaaca jirka oo ay weheliso kiimoterabi ay si weyn u wanaajisay badbaadada aan dhacdooyinka lahayn (EFS) ee marxaladda neoadjuvant marka la barbar dhigo kiimoterabiga oo keliya. Intaa waxaa dheer, neoadjuvant immunotherapy waxay sidoo kale hoos u dhigi kartaa heerka soo noqnoqda iyada oo la hagaajinayo heerka jawaab celinta dhamaystiran ee pathological (pCR) ee bukaanada, taas oo yareyneysa suurtagalnimada soo noqoshada qalliinka kadib.
2. Perioperative immunotherapy (neoadjuvant + adjuvant): Habkan, immunotherapy waxaa la maamulaa ka hor iyo ka dib qalliinka si loo kordhiyo saamaynta antitumor iyo in dheeraad ah ka saaro dhaawacyada ugu yar ee haraaga qalliinka ka dib. Hadafka ugu muhiimsan ee qaabkan daawaynta ayaa ah in la wanaajiyo badbaadada muddada dheer iyo daaweynta heerarka bukaanka burooyinka iyadoo la isku darayo immunotherapy marxaladaha neoadjuvant (kahor) iyo adjuvant (ka dib qaliinka). Keykeynote 671 waa wakiilka qaabkan [2]. Sida tijaabada kaliya ee la kantaroolay ee la kala soocay (RCT) oo leh EFS togan iyo OS dhamaadka dhibcood, waxay qiimeysay waxtarka palizumab oo ay weheliso kiimoterabiga marxaladda qalliinka dib-u-soo-celinta Ⅱ, ⅢA, iyo ⅢB (N2) bukaannada NSCLC. Marka la barbardhigo kiimoterabiga oo keliya, pembrolizumab oo ay weheliso kiimoterabi waxay kordhisay EFS dhexdhexaadka ah 2.5 sano waxayna hoos u dhigtay khatarta horumarinta cudurrada, soo noqoshada, ama dhimashada 41%; KEYNOTE-671 sidoo kale waxay ahayd daraasaddii ugu horreysay ee tallaalka tallaalka si loo muujiyo faa'iidada guud ee badbaadada (OS) ee NSCLC ee dib loo soo celin karo, iyadoo 28% ay hoos u dhigtay halista dhimashada (HR, 0.72), oo ah guul muhiim u ah neoadjuvant iyo adjuvant immunotherapy ee la shaqeyn karo heerka hore ee NSCLC
3. Adjuvant immunotherapy oo kali ah: Habkan, bukaanku ma helin daawaynta dawada qalliinka ka hor, iyo immunodrugs ayaa la isticmaalay qalliinka ka dib si looga hortago soo noqoshada burooyinka haraaga ah, taas oo ku habboon bukaanada leh khatarta soo noqoshada. Daraasada IMpower010 waxay qiimeysay waxtarka adjuvant adjuvant attilizumab oo ka soo horjeeda daaweynta ugu habboon ee bukaanka leh marxaladda gebi ahaanba dib loo soo celiyay IB ilaa IIIA (AJCC 7th edition) NSCLC [3]. Natiijooyinku waxay muujiyeen in daawaynta isku-dhafan ee attilizumab ay si weyn u daba-dheeraatay badbaadada cudur-la'aanta (DFS) ee bukaannada togan ee PD-L1 ee heerka ⅱ ilaa ⅢA. Intaa waxaa dheer, daraasadda KEYNOTE-091 / PEARLS waxay qiimeysay saameynta pembrolizumab sida daaweynta isku-dhafka ah ee bukaannada gebi ahaanba la soo saaray ee leh marxaladda IB ilaa IIIA NSCLC [4]. Pabolizumab ayaa si weyn ugu dheeraaday guud ahaan dadweynaha (HR, 0.76), iyada oo dhexdhexaadinta DFS ee 53.6 bilood ee kooxda Pabolizumab iyo 42 bilood ee kooxda placebo. Koox-hoosaadka bukaanada leh dhibcaha saamiga saamiga buro PD-L1 (TPS) ≥50%, inkasta oo DFS ay ku dheeraatay kooxda Pabolizumab, farqiga u dhexeeya labada kooxood ma ahayn mid muhiim ah sababtoo ah cabbirka saamiga yar, iyo dabagal dheer ayaa loo baahan yahay si loo xaqiijiyo.
Marka loo eego haddii immunotherapy lagu daro daawooyinka kale ama tallaabooyinka daweynta iyo qaabka isku dhafan, barnaamijka neoadjuvant immunotherapy iyo adjuvant immunotherapy waxaa loo qaybin karaa saddexda nooc ee soo socda:
1. Hal immunotherapy: Noocan daaweynta waxaa ka mid ah daraasado ay ka mid yihiin LCMC3 [5], IMpower010 [3], KEYNOTE-091/PEARLS [4], BR.31 [6], iyo ANVIL [7], oo lagu garto isticmaalka hal dawooyinka difaaca jirka sida (cusub) daaweynta adjuvant.
2. Isku darka immunotherapy iyo kiimoterabiga: Daraasadaha noocan oo kale ah waxaa ka mid ah KEYNOTE-671 [2], CheckMate 77T [8], AEGEAN [9], RATIONALE-315 [10], Neotorch [11], iyo IMpower030 [12]. Daraasadahani waxay eegeen saamaynta isku darka immunotherapy iyo chemotherapy ee xilliga qalliinka.
3. Isku-darka difaaca jirka iyo hababka kale ee daaweynta: (1) Isku-darka difaaca jirka: Tusaale ahaan, cytotoxic T lymphocyte-ku xiran antigen 4 (CTLA-4) ayaa lagu daray baaritaanka NEOSTAR [13], lymphocyte activation gene 3 (LAG-3) antibody ayaa la isku daray NEO-Predict-Lung, iyo TIM-ga oo isku dhafan qaab-dhismeedka IT-ga. Tijaabada SKYSCRAPER 15 Daraasado ay ka mid yihiin isku-darka TIGIT antibody [15] waxay xoojiyeen saamaynta ka-hortagga buro iyadoo la isku daray daawooyinka difaaca. (2) Lagu daray daaweynta shucaaca: tusaale ahaan, duvaliumab oo lagu daray daaweynta shucaaca stereotactic (SBRT) waxaa loogu talagalay in lagu wanaajiyo saameynta daweynta ee hore ee NSCLC [16]; (3) Isku darka daawooyinka anti-angiogenic: Tusaale ahaan, daraasadda EAST ENERGY [17] waxay sahamisay saameynta isdhexgalka ee ramamab oo ay weheliso immunotherapy. Sahanka hababka difaaca jirka ee badan ayaa muujinaya in habka codsiga ee immunotherapy ee muddada qalliinka aan weli si buuxda loo fahmin. Inkasta oo immunotherapy oo keliya ay muujisay natiijooyin wax ku ool ah oo ku saabsan daaweynta qalliinka, iyada oo la isku darayo kiimoterabiga, daaweynta shucaaca, daaweynta antiangiogenic, iyo kuwa kale ee xakameynaya isbaarada difaaca sida CTLA-4, LAG-3, iyo TIGIT, cilmi-baarayaashu waxay rajeynayaan inay sii wanaajiyaan waxtarka immunotherapy.
Weli ma jiro gabagabo ku saabsan habka ugu habboon ee daaweynta tallaalka ee NSCLC hore ee la shaqeyn karo, gaar ahaan haddii immunotherapy perioperative marka la barbar dhigo immunotherapy neoadjuvant oo keliya, iyo haddii tallaalka difaaca jirka ee dheeraadka ah uu keeni karo saameyno dheeraad ah oo dheeraad ah, weli waxaa jira la'aanta natiijooyinka tijaabada isbarbardhigga tooska ah.
Forde iyo al. loo adeegsaday falanqaynta miisaanka sahaminta si loo qiyaaso saamaynta tijaabooyinka la kantaroolay ee la kala soocay, oo la hagaajiyay jaangooyooyinka aasaasiga ah iyo astaamaha cudurka ee dadyowga kala duwan ee daraasadda si loo yareeyo saamaynta jaahwareerka ah ee arrimahan, taasoo ka dhigaysa natiijooyinka CheckMate 816 [1] iyo CheckMate 77T [8] is barbar dhig. Waqtiga daba-galka dhexdhexaadka ah wuxuu ahaa 29.5 bilood (CheckMate 816) iyo 33.3 bilood (CheckMate 77T), siday u kala horreeyaan, iyagoo bixinaya wakhti ku filan oo dabagal ah si loo ilaaliyo EFS iyo tallaabooyinka kale ee waxtarka leh.
Falanqaynta miisaanka leh, HR ee EFS waxay ahayd 0.61 (95% CI, 0.39 ilaa 0.97), oo soo jeedinaysa 39% khatarta hoose ee soo noqoshada ama dhimashada ee nabuliumab isku-darka kooxda kemotherabi (CheckMate 77T) marka la barbar dhigo neoadjuvant naburiumab isku darka kemoterapiska (1) . Nebuliyuzumab perioperative oo lagu daray kooxda kemotherabi waxay muujisay faa'iido dhexdhexaad ah dhammaan bukaannada marxaladda asaasiga ah, saameyntuna waxay ahayd mid aad u muuqata bukaanada leh wax ka yar 1% buro PD-L1 (49% hoos u dhigista khatarta soo noqoshada ama dhimashada). Intaa waxaa dheer, bukaanada ku guuldareystay inay gaaraan pCR, kooxda naburiumab ee isku dhafka kemotherabi waxay muujiyeen faa'iido weyn EFS (35% hoos u dhigista khatarta soo noqoshada ama dhimashada) marka loo eego kooxda neoadjuvant nabuliumab isku darka kemotherabi. Natiijooyinkani waxay soo jeedinayaan in qaabka immunotherapy perioperative uu ka faa'iido badan yahay qaabka immunotherapy neoadjuvant oo kali ah, gaar ahaan bukaanada qaba muujinta PD-L1 ee hooseeya iyo hadhaaga burooyinka ka dib daaweynta bilowga ah.
Si kastaba ha ahaatee, qaar ka mid ah isbarbardhigga aan tooska ahayn (sida falanqaynta-meta) ma muujin farqi weyn oo u dhexeeya badbaadada u dhexeeya immunotherapy neoadjuvant iyo immunotherapy perioperative [18]. Falanqaynta maadada ee ku salaysan xogta bukaan-socodka shakhsi ahaaneed ayaa lagu ogaaday in immunotherapy perioperative iyo neoadjuvant immunotherapy ay natiijooyin isku mid ah ka heleen EFS ee labadaba pCR iyo kuwa aan PCR-hoosaadka ee bukaannada qaba heerka hore ee NSCLC [19]. Intaa waxaa dheer, wax ku biirinta marxaladda difaaca jirka ee adjuvant, gaar ahaan ka dib marka bukaanku gaaro pCR, ayaa weli ah arrin muran leh oo rugta caafimaadka ah.
Dhawaan, Maamulka Cunnada iyo Dawooyinka ee Mareykanka (FDA) Guddiga La-talinta Daawooyinka Oncology ayaa ka wada hadlay arrintan, iyaga oo xoogga saaraya in doorka gaarka ah ee tallaalka difaaca jirka uusan wali caddayn [20]. Waxaa laga dooday in: (1) Way adag tahay in la kala saaro saamaynta marxalad kasta oo daawaynta ah: sababtoo ah barnaamijka perioperative wuxuu ka kooban yahay laba weji, neoadjuvant iyo adjuvant, way adagtahay in la go'aamiyo tabaruca shakhsi ahaaneed ee weji kasta saamaynta guud, taas oo adkeynaysa in la go'aamiyo marxaladda muhiimka ah, ama haddii labada weji ay u baahan yihiin in la fuliyo isku mar; (2) Suurtagalnimada daaweynta xad-dhaafka ah: haddii tallaalka difaaca jirka uu ku lug leeyahay labada weji ee daaweynta, waxay keeni kartaa bukaanada inay helaan daaweyn dheeraad ah oo ay kordhiso khatarta waxyeellooyinka; (3) Culeyska daawaynta oo kordhay: Daawaynta dheeraadka ah ee marxaladda daaweynta adjuvant waxay u horseedi kartaa culays daweyn oo sarreeya oo bukaannada ah, gaar ahaan haddii ay jirto hubanti la'aan ku saabsan waxtarkeeda guud ee waxtarka. Iyadoo laga jawaabayo dooda sare, si loo soo saaro gabagabo cad, si adag loo qaabeeyey tijaabooyin la xakameynayo ayaa loo baahan yahay xaqiijin dheeraad ah mustaqbalka.
Waqtiga boostada: Dec-07-2024